How sharks and camels could be the keys to curing brain diseases
It's all in the antibodies

Main image credit: Jakob Owens on Unsplash
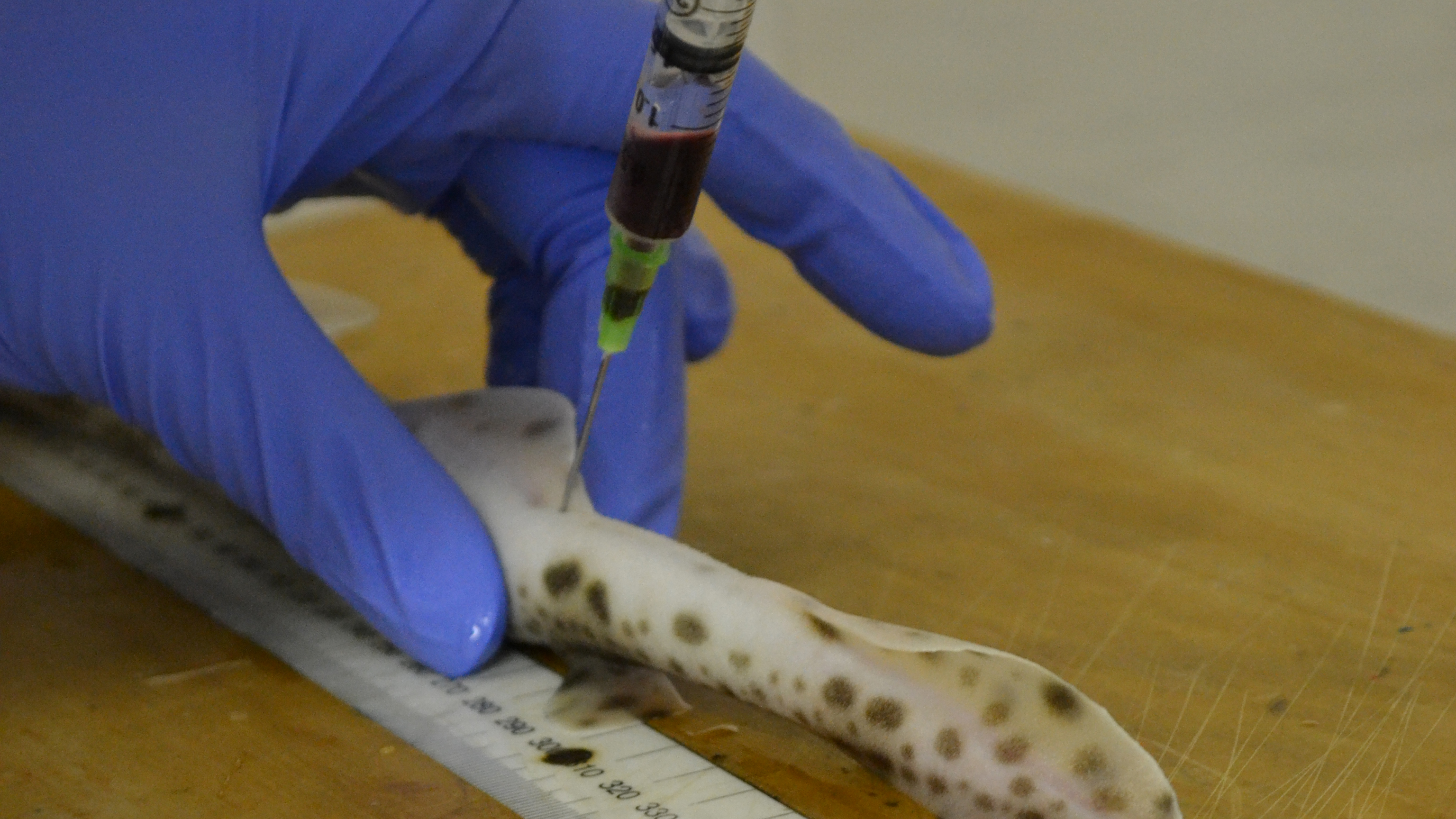
“I bleed the sharks every month or two,” aren’t the words you expect to hear from an assistant professor at Maryland University. But that’s exactly what Dr Helen Dooley does in her work as an immunologist, visiting the Institute of Marine and Environmental Technology in Baltimore to collect samples for her research.
She, and other academics around the world, believe that proteins harvested from shark blood could be the key to diagnosing and treating diseases that are hard to target with existing drugs.
The reason the shark blood is useful is that it contains antibodies – proteins produced by an organism's immune system in reaction to foreign bodies – that are a tenth of the size of those found in humans. They can therefore penetrate certain barriers that “large and clunky” human antibodies can’t, like the ones between your blood and your brain, Dooley explains.
Scientists therefore believe the antibodies could be used to help diagnose and treat brain diseases like Alzheimer’s and brain cancer. So how does it work? And what are the challenges that Dooley and others are facing?
- Do you have a brilliant idea for the next great tech innovation? Enter our Tech Innovation for the Future competition and you could win up to £10,000!
The anti-antibody barrier
The blood-brain barrier is very tight, which means human antibodies can’t cross it. That’s by design: an antibody attacking healthy brain tissue would be a disaster. But it also means that finding proteins that can target diseases in the brain is notoriously difficult.
In theory, scientists should be able to use shark blood to manufacture a protein that can bind to specific targets in the brain, and it all starts by immunizing a shark with whatever target you’re aiming for. When a shark’s immune system detects the marker, it will produce antibodies against it.
Sign up for breaking news, reviews, opinion, top tech deals, and more.
“If it’s a brain tumor, we would immunize it with things found on the surface of that brain tumor,” Dooley explains. “We then look at the antibodies that the shark is making, and use synthetic versions of those to treat people.”

Scientists can then 'clone' the antibodies, using a method called a Polymerase Chain Reaction (PCR) to generate multiple copies of the antibody’s DNA – almost like a “genetic copy and paste”, Dooley says. Next, the resultant protein is put into a bacteria, which acts like a “factory” generating lots of copies of it.
All the while, Dooley is changing the shark protein to make it “more like a human” protein: “We find a protein that it looks a lot like in humans, and then basically you can tweak the proteins, mutate the genetic sequence to make it look more like that [human] protein.”
The main hurdle, she says, is working out just how much to modify the protein. “Nobody has really done that before: how less ‘shark’ does it have to be, and how much more ‘human’ does it have to be? That’s probably the biggest challenge.”
The research is still in its early stages, but scientists have had most success when they create a protein called a nanobody, which resembles a small part of the animal antibody. Philadelphia-based biotech firm Ossianix has created a protein that looks like a section of a shark antibody, and which can bind to a receptor that controls access to the blood-brain barrier. Ossianix believes that, by activating the receptor, the protein might allow antibodies – and drugs – that can’t usually penetrate the barrier to pass through.
A cure from camels?
Treating brain diseases is perhaps the most exciting possibility for the proteins, but they can also be useful elsewhere in the body: several clinical trials are underway to test the effectiveness of animal-derived proteins on diseases ranging from arthritis to psoriasis. And it’s not just sharks that could help provide new treatments – camels and llamas have similarly small antibodies.
Last year, a Belgian company ran a trial of caplacizumab, derived from a llama protein, in patients with a rare clotting disease called thrombotic thrombocytopenic purpura, and has already applied for a licence to sell the drug in Europe.
They might be useful outside of treatment, too. Dooley’s main area of interest is how the proteins can aid imaging of tumors and other diseases. Their size allows them to penetrate tissues better, and if you attach a radioactive isotope to them then they’ll show up on a PET scan. You could therefore design a protein to bind to a tumor, and then scan the area to see the tumor’s exact size and location.

“Because they’re so small they get cleared from the body fast if they’re not bound to something,” Dooley explains, which is helpful. “For imaging, you want to get rid of the stuff that isn’t bound because it brings your background [reading] down, so you can find smaller tumors.”
In the past, Dooley has also worked with defense organizations around the world to fight outbreaks of diseases like Ebola. Because the proteins are smaller than others, they’re more stable, and therefore easier to transport. “You don’t have to refrigerate them if you’re shipping them, so you can create better diagnostics to test for things like Ebola outbreaks,” she says. ”You can just put them in a cool box and ship them off on the back of a motorbike.”
The obstacles to acceptance
The potential of proteins derived from shark, camel and llama blood is clear, but they’re still a way off making it into mainstream medicine. Dooley doubts that they will ever replace “blockbuster drugs” because they’re cleared faster from the body, and therefore may not be effective as long-term treatments. The fact that they’re derived from animals means it’s also difficult to get approval for drugs, Dooley says.
“People get nervous when you say you’re going to put something from an animal into a human.“
Dr Helen Dooley
“People get nervous when you say you’re going to put something from an animal into a human. Traditionally, we have done that: if you’re bitten by a snake they’ll give you anti serum that’s generated in a horse or goat. It’ll keep you alive, but it’ll make you very sick. That’s something we want to avoid.”
Finally, there are the research challenges: Dooley and others still need to prove that these proteins can cross the blood-brain barrier, for one. But if they manage it – and if the work of other scientists continues at its current pace – then the implications could be massive.
“I can see ways where we can help, [especially] with diagnosis or imaging,” Dooley says. “We’re excited. I think the potential is pretty huge.”
TechRadar's Next Up series is brought to you in association with Honor
