This battery breakthrough could help next-gen gadgets last longer
A future where batteries never stop working?
Sign up for breaking news, reviews, opinion, top tech deals, and more.
You are now subscribed
Your newsletter sign-up was successful
German researchers at the Technical University of Munich (TUM) have been looking into what is making your batteries die so soon.
Specifically, the researchers have been looking at lithium-ion batteries, the type you'll find in your phone and laptop, and even in some planes and electric cars. These lose charging capacity over time, which is why your device's battery life diminishes after a certain amount of use.
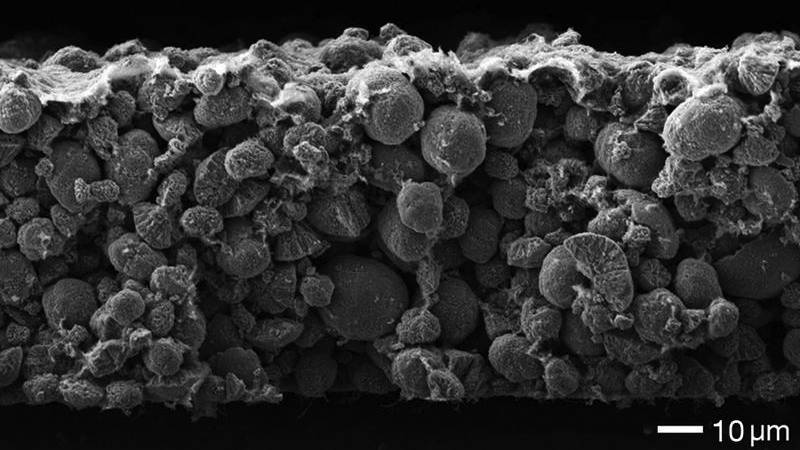
The university explains that lithium-ion batteries with graphite anodes (an electrode through which a conventional current flows), lose most of their capacity during the very first charging cycle, largely due to the active lithium component being consumed when the "pacifying layer on the anode" forms.
This pacifying layer protects the battery electrolyte from decomposition at the anode.
Every charge thereafter also reduces capacity, although the drop is barely noticeable. Capacity can also be lost even without charging the battery: simply storing a battery – especially in above-room-temperature environments, causes a reduction in capacity.
Aging batteries
Researchers at TUM say that while others haven't been able to find a definitive answer as to why the batteries age in such a matter, they have come a step closer in "closing this knowledge gap".
The team used electrochemical investigations, as well as 'X-ray diffraction' and temperature effects, during experiments.
Sign up for breaking news, reviews, opinion, top tech deals, and more.
The team found that capacity loss occurred due to active lithium consumption at higher temperatures, while high voltages increased the conductivity of the pacifying layer and therefore the decomposition of the electrolyte.
With these findings, the researchers believe that certain additives can be used to change the way the pacifying layer is formed so that the active lithium isn't affected, resulting in battery capacity being maintained.
"Using our insights, now individual processes can be improved," says Irmgard Buchberger, PhD student at the Department of Electrochemistry at TUM. We'll see if this knowledge makes it to our device batteries any time soon.
- Unlike your phone battery, our Holiday Gift Guide never runs out of good deals.
Image credit: Irmgard Buchberger/TUM