FDA approves 'bionic' eye, brings partial vision to the blind
$150K technology initially available in only seven hospitals
It may not quite be the sophisticated technology used to turn Steve Austin into The Six Million Dollar Man back in the '70s, but a new "bionic" eye can restore at least partial vision to those without it.
The New York Times reported Thursday that the Food and Drug Administration (FDA) approved an initial treatment for new technology known as an "artificial retina."
Like something out of science fiction, the artificial retina is essentially a bionic eye that restores limited vision for certain types of blindness.
Considered a milestone in vision research, the technology will allow blind people to see crosswalks, better detect people or vehicles and in some cases even view letters or numbers, provided the markings are big enough.
How it works
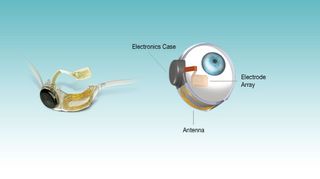
Marketed as the Argus II, the artificial retina uses a pair of glasses outfitted with a camera and video processor, all connected to a sheet of electrodes implanted inside a patient's eye.
The system doesn't restore actual vision, but instead helps those wearing it to "see" outlines, particularly for objects with a high degree of contrast between light and dark.
"Without the system, I wouldn't be able to see anything at all, and if you were in front of me and you moved left and right, I'm not going to realize any of this," a 74-year-old retired electrician who participated in early clinical trials told the Times.
Get daily insight, inspiration and deals in your inbox
Get the hottest deals available in your inbox plus news, reviews, opinion, analysis and more from the TechRadar team.
Ophthalmologist Dr. Mark S. Humayun spent 20 years developing the technology behind Argus II at the University of Southern California.
Limited availability
Second Sight Medical Products received FDA approval Thursday to treat patients with severe retinitis pigmentosa, a condition involving deterioration in the eye's photoreceptor cells.
Artificial retina technology will be available at only seven hospitals initially, where it's expected to cost upwards of $150,000 in addition to training and surgery costs.
The report notes that 100,000 Americans suffer from retinitis pigmentosa, but less than 15,000 of them age 25 and older are expected to qualify initially.
The Argus II received European approval back in 2011, where it's also being used to treat severe blindness from outer retinal degeneration.
Most Popular


